[ad_1]
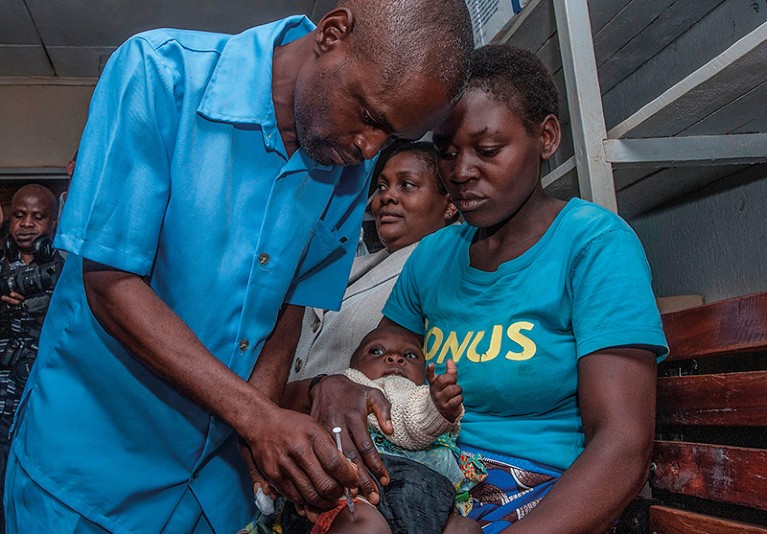
Ghana, Kenya and Malawi took half in a pilot examine of the RTS,S vaccine in 2019.Credit score: Amos Gumulira/AFP through Getty
John Bawa, who leads vaccine implementation in Africa on the international non-profit group PATH in Accra, has been working for greater than a decade on the primary vaccine towards malaria. And he has develop into used to listening to the identical query: “The place is your vaccine?” So, final yr, when the World Well being Group (WHO) really useful the usage of the vaccine, generally known as RTS,S and marketed as Mosquirix, in kids dwelling in international locations hardest hit by the illness, “it was an important aid for us”, he says. “Now I’ve my vaccine.”
The WHO’s suggestion was a historic milestone. RTS,S took 30 years to develop, and isn’t solely the primary malaria vaccine, but additionally the primary vaccine for any parasitic illness. Though the efficacy of the shot is modest — about 50% within the first yr1 — it’s anticipated to save lots of tens of 1000’s of lives every year. One examine2 estimated that, if the vaccine had been rolled out in international locations with the very best burden of malaria, it might stop 5.3 million instances and 24,000 deaths in younger kids every year.
A part of Nature Outlook: Youngsters‘s well being
However reaping that profit will take time. Up to now, a couple of million kids have acquired a number of doses of the vaccine in a pilot examine in Ghana, Kenya and Malawi. That’s only a fraction of the 25 million kids in additional than 30 international locations who want it. It could possibly be years earlier than a lot of these international locations get their first doses.
Specialists estimate that demand might be 80 million to 100 million doses per yr. The vaccine’s producer GlaxoSmithKline (GSK), a pharmaceutical firm based mostly in Brentford, UK, has promised to ship 18 million doses over the following 3 years. For the individuals who have watched this vaccine hit roadblock after roadblock throughout its lengthy interval of improvement, the availability issues come as a disappointment.
However Dyann Wirth, a malaria researcher on the Harvard T.H. Chan College of Public Well being in Boston, Massachusetts, is making an attempt to look on the brilliant facet. Having some vaccine is a lot better than having none. RTS,S approval “modified the dialog about whether or not vaccines had been possible for malaria”, she says. And that can pave the best way for higher vaccines.
The lengthy delay
Public-health employees have made spectacular features towards malaria in current many years. Between 2000 and 2019, international deaths attributable to malaria fell by round 40%. But the illness stays one of many prime causes of loss of life amongst kids. In 2020, the world noticed 241 million instances of malaria and 627,000 deaths. Africa accounted for round 95% of the instances and deaths, and 80% of these deaths had been kids below 5.
RTS,S works by focusing on a portion of the circumsporozoite protein on the floor of the malaria parasite. The thought is {that a} vaccinated particular person will generate antibodies and kill off the parasite earlier than it may well enter crimson blood cells. However the vaccine doesn’t grant good safety. Within the first yr after vaccination, it’s about 50% efficient in stopping scientific instances of malaria in kids aged 5 to 17 months1. After 4 years, efficacy drops to 36% for scientific episodes and 32% for extreme malaria. The vaccine has additionally been examined in youthful infants aged between 6 and 12 weeks, however efficacy was decrease and the advantages weren’t deemed important sufficient to justify its use on this age group.
These efficacy numbers are disappointing in comparison with vaccines for measles or polio, that are greater than 90% protecting. However “once we put that within the spectrum of how efficient our different interventions are at stopping extreme malaria, it’s not as unhealthy because it appears”, says Joshua Yukich, an epidemiologist at Tulane College in New Orleans, Louisiana. “It’s not like giving an individual a mattress web prevents 100% of extreme malaria episodes.” And the hope is that the vaccine might be added to different interventions which might be already in place. In actual fact, when researchers mixed the vaccine with medicines to forestall malaria in areas with seasonal transmission, the mixture provided a lot increased safety towards the illness than both technique alone3 — round 60%.
The section III trial for RTS,S wrapped up in 2014. For many vaccines, that is the ultimate step earlier than approval and roll-out. However the vaccine wanted to cross the WHO’s prequalification programme, which certifies the security and efficacy of medicines and vaccines destined for low- and middle-income international locations. When the WHO’s advisory committee on immunization and malaria met to debate the vaccine, some members had issues.
The examine had not been capable of decide the impression of RTS,S on mortality. What’s extra, researchers had picked up instances of meningitis in kids who had acquired the vaccine, and the WHO committee members needed to make sure there wasn’t a causal hyperlink. In addition they questioned the feasibility of rolling out a four-dose vaccine in Africa. The timing of the doses doesn’t totally line up with these for different childhood vaccines, and reaching kids after their first yr of life will be tough. “Folks had been very sceptical that an African health-care system was going to have the ability to ship that vaccine in a manner that was price it,” Yukich says.
So reasonably than giving RTS,S a full suggestion, the WHO determined to green-light a large-scale pilot examine in 2019 in Ghana, Kenya and Malawi. That examine doesn’t formally wrap up till 2023, however by October 2021 the WHO and its advisers had sufficient information to see that the vaccine was secure, uptake was good and implementation regarded possible (see go.nature.com/3ilss7v).
Working a pilot examine made sense on the time, Wirth says — this was the primary vaccine for parasitic illness ever utilized in individuals, in spite of everything. However she says it’s truthful to query whether or not the proper stability was struck between security and urgency. “Knowledge from the section II trials is kind of the identical as the info from the pilot research,” she says. The section II outcomes had been revealed greater than a decade in the past. Throughout that interval “tons of of 1000’s of youngsters suffered critical malaria, and lots of of them died”.
Roll-out woes
The WHO’s endorsement is a crucial step in getting RTS,S to the kids who want it, however most of these kids received’t profit any time quickly. When the WHO initially determined to launch the pilot examine in 2015, GSK closed their RTS,S manufacturing plant in Wavre, Belgium. It didn’t reopen till 2019, and ramping up manufacturing will take time.
GSK has promised to ship 4 million doses in 2023, 6 million in 2024 and eight million in 2025. By 2026, GSK’s plant will produce 15 million doses a yr. “That’s all the ability can produce,” says Thomas Breuer, chief international well being officer at GSK. However that quantity is a fraction of the anticipated demand.
“There are 40 million youngsters born in sub-Saharan Africa alone in malarious areas of average to excessive transmission,” says Adrian Hill, director of the Jenner Institute on the College of Oxford, UK, and RTS,S requires 4 doses. “That’s 160 million doses a yr.” The WHO predicts that demand might be decrease — within the realm of 100 million doses per yr. However each estimates symbolize way more vaccine than GSK plans to supply.
GSK has introduced that, by 2028, it can switch the know-how to fabricate RTS,S to Bharat Biotech, a biotechnology firm in Hyderabad, India. This could assist to bolster provides, however there’s a catch. RTS,S consists of two components: the antigen (RTS,S) and an adjuvant referred to as AS01E, which helps to spice up the immune response. Bharat Biotech will manufacture the antigen, however GSK will nonetheless provide the adjuvant. AS01E comprises a sort of chemical referred to as a saponin — particularly, “a specific saponin you’ll be able to solely extract from a tree referred to as Quillaja saponaria, which grows primarily in Chile”, Hill says. GSK has promised to provide as much as 30 million doses of adjuvant per yr. “If extra is required, we’ll discover it,” Breuer says. He factors out that the overwhelming majority of this saponin is used for different functions, akin to in cosmetics, and could possibly be diverted. “The adjuvant won’t be the bottleneck,” he says.
Till then, nonetheless, the restricted provides of vaccine that do exist should be rigorously distributed. That job falls to Gavi, the Vaccine Alliance, in Geneva, Switzerland, which helps to vaccinate kids on this planet’s poorest international locations. In December 2021, Gavi’s board authorized US$155.7 million for the roll-out of RTS,S from 2022 to 2025. Nations that need the vaccine can apply for funding from Gavi, which can contemplate a wide range of elements, together with every nation’s malaria burden and capability to deploy the vaccine. Nations that obtain vaccines by way of Gavi can pay a variable portion of the associated fee, relying on their revenue.
Solely the three international locations that piloted the vaccine had been eligible for the primary spherical of funding. “We need to be sure that we will get ample provides to them in order that they will proceed their programmes with out having any pause,” says Stephen Sosler, head of vaccine programmes at Gavi. The subsequent spherical will shut in January 2023. As of late November, 21 international locations had expressed curiosity in making use of. Gavi is working laborious to attempt to get these international locations their first doses by the tip of 2023. As a result of provides are so restricted, Gavi will take further care to be sure that every dose has the utmost impression and that there isn’t any waste, Sosler says.
Options on trial
Having a number of vaccines would assist to ease provide constraints. In July, the biotechnology firm BioNTech, based mostly in Mainz, Germany, introduced plans to launch trials of an mRNA vaccine for malaria by the tip of this yr. One other malaria vaccine, being developed on the College of Oxford, referred to as R21, is already in section III scientific trials and, if confirmed to be secure and efficient, it could possibly be deployed as quickly as 2023. R21 makes use of the identical antigen as RTS,S, however the outcomes of R21’s section II trials, launched in September4, counsel that it is perhaps simpler. In a trial of about 400 kids in Burkina Faso, 4 doses offered round 75% safety towards scientific instances of malaria after 12 months.
Extra from Nature Outlooks
That quantity sounds spectacular, however whether or not it can maintain up within the section III trial isn’t clear. The section II examine occurred in West Africa, the place malaria transmission is seasonal. “There’s a peak of malaria in September, October and November. We had been vaccinating in June and July,” says Hill, simply earlier than the malaria season hit. However, “what is going to occur whenever you go to a spot the place transmission is over 12 months of the yr?” says Halidou Tinto, the regional director on the Institute of Analysis in Well being Sciences (IRSS) in Nanoro, Burkina Faso.
If R21 is authorized, it might drastically enhance malaria vaccine provides. The world’s largest vaccine producer — the biopharmaceuticals firm Serum Institute of India in Pune — has already dedicated to producing greater than 200 million doses per yr, a amount that Tinto calls “superb”.
In fact, getting any malaria vaccine to low-income international locations would require a hefty and sustained funding. “We hoped that the form of help that COVID had, with all of the funding, with all of the curiosity, would have come on board,” Bawa says. That hasn’t occurred, but. However he nonetheless hopes it can. “The struggle towards malaria is a world struggle,” he says. If the world can eradicate malaria, “posterity would always remember us”.
[ad_2]


